Antimicrobial peptides (AMPs) are components of the innate immune system in virtually all species. Most of them are thought to protect the host by permeabilizing the membrane of pathogenic bacteria. This is proposed to proceed via either pore formation or detergent-like membrane destabilization. Several years ago we studied the configuration and energetics of an alamethicin monomer in implicit membrane (Ref. 1). We found two configurations of very similar energy, one tilted interfacial and the other more deeply inserted, almost transmembrane. Application of voltage stabilized the deeply inserted configuration.
We used implicit and explicit solvent modeling to study the interaction of several antimicrobial peptides, such as melittin, magainin and piscidin, with membrane pores. We found that these peptides bind more strongly to pores than to flat membranes, consistent with the idea that they stabilize pores (Ref. 2). We proposed that the mechanism for this may be the imperfect amphipathicity of these peptides. At the same time we performed explicit solvent simulations of these peptides (Ref. 3) and several mutants thereof (Ref. 4) to see the details of the pore structures and the determinants of toroidal vs cylindrical pore formation.
More recently we extended these simulations to the multi-microsecond timescale (Refs 10,11,14,15). We have also studied protegrin, a beta-hairpin peptide, and many of its analogues in both implicit and explicit membranes (Refs 5,8,9,12,13) and analyzed the energetics of alamethicin oligomer formation (Ref. 7). A large scale comparative study of dozens of helical peptides revealed correlations between their structure, their binding membranes and pores, and their activity (Ref. 6).
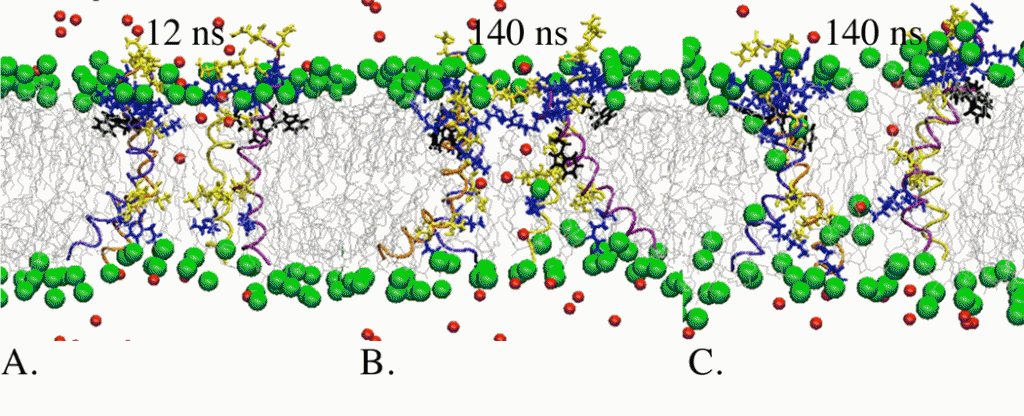
From (Ref. 3) A melittin tetramer interacting with lipid headgroups and Cl− ions in a constrained cylindrical pore (A) and toroidal pores (B and C). Polar residues are shown as yellow licorice, charged residues as blue licorice, W19 as black licorice, phosphocholines as green balls, ions as red balls, lipid tails as gray lines, and peptides as blue, orange, yellow and purple ribbons
References
- Mottamal, M., Lazaridis, T. “Voltage-dependent energetics of alamethicin monomers in the membrane”, Biophys. Chem. , 122:50-7 (2006)
- Mihajlovic, M., Lazaridis, T. “Antimicrobial peptides bind more strongly to membrane pores”, BBA-Biomembranes , 1798:1494-1502 (2010)
- Mihajlovic, M., Lazaridis, T. “Antimicrobial Peptides in Toroidal and Cylindrical Pores”, BBA-Biomembranes , 1798:1485-1493 (2010)
- Mihajlovic, M., Lazaridis, T. “Charge Distribution and Imperfect Amphipathicity Affect Pore Formation by Antimicrobial Peptides”, BBA-Biomembranes , 1818:1274-83 (2012)
- Lazaridis, T., He Y., Prieto L. “Membrane interactions and pore formation by the antimicrobial peptide protegrin”, Biophysical J, 104:633-42 (2013)
- He Y., Lazaridis, T. “Activity Determinants of Helical Antimicrobial Peptides: A Large-scale Computational Study”, PLOS One, 8(6): e66440 (2013)
- Rahaman A., Lazaridis, T. “A thermodynamic approach to alamethicin pore formation”, BBA Biomembranes 1838:98 (2014)
- Prieto L., He Y., Lazaridis T. “Protein arcs may form stable pores in membranes”, Biophys J, 106:154-161 (2014)
- Lipkin R.B., Lazaridis T. “Implicit Membrane Investigation of the Stability of Antimicrobial Peptide beta-barrels and arcs”, J Mem Biol, 248:469-86 (2015)
- Leveritt J.M. III, Pino-Angeles A., Lazaridis T. “The Structure of a Melittin-Stabilized Pore”, Biophys J, 108:2424-6 (2015)
- Pino-Angeles A.,Leveritt J.M. III, Lazaridis T. “Pore Structure and Synergy in Antimicrobial Peptides of the Magainin Family”, PLOS Comp. Biol. 12:e1004570 (2016)
- Lipkin R., Pino-Angeles A., Lazaridis T. “Transmembrane Pore Structures of beta-Hairpin Antimicrobial Peptides by All-Atom Simulations”, J. Phys. Chem. B, 121:9126-40 (2017)
- Rodnin M.V., Vasquez-Montes V., Nepal B., Ladokhin A.S., Lazaridis T., “Experimental and Computational Characterization of Oxidized and Reduced Protegrin Pores in Lipid Bilayers”, J. Mem. Biol. 253:287-98 (2020)
- Pino-Angeles A., Lazaridis T. “Effects of peptide charge, orientation, and concentration on melittin transmembrane pores”, Biophysical J, 114:2865 (2018)
- Sepehri A., PeBenito L, Pino-Angeles A., Lazaridis T. “What Makes a Good Pore Former: A Study of Synthetic Melittin Derivatives”, Biophys. J., 118:1901-13 (2020)
